Cryoablation vs Radiofrequency Ablation and the Impact on Atrial Fibrillation Treatments
Cryoablation and radiofrequency (RFA) are two common ablation modalities for treating atrial fibrillation (AF), problematic tissue treatment, and other conditions. RFA is the long-standing method for ablation therapy, however, cryoablation use is increasing, especially for AF treatments, sparking discussions and studies around cryoablation vs radiofrequency ablation. While both are minimally invasive and effective, they […]
Pulsed Field Ablation vs Radiofrequency Ablation: A Comparison of Two Ablation Therapies
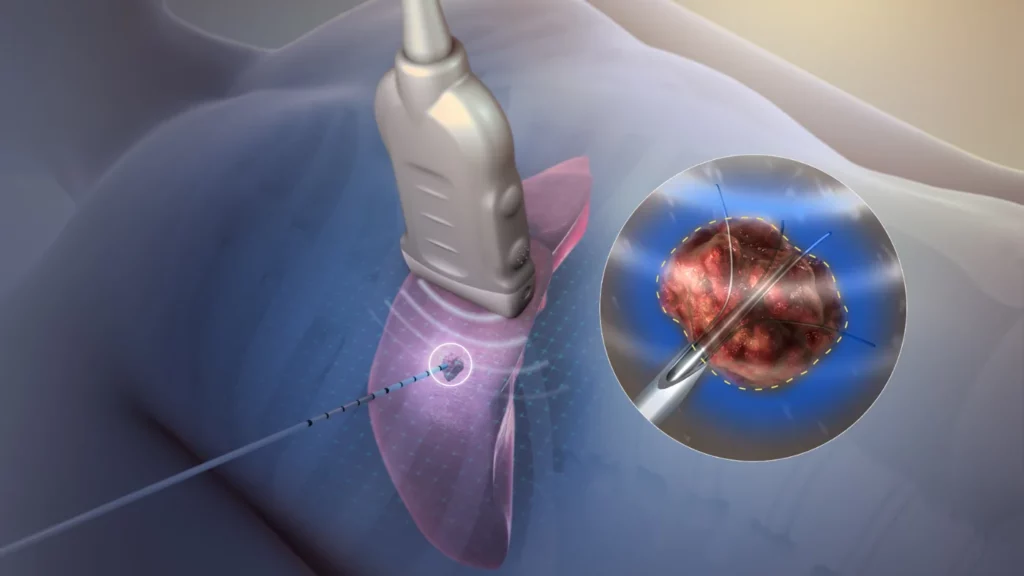
Radiofrequency, a common energy used in ablation therapies, is used for treating damaged, diseased, or malfunctioning tissue with minimally invasive procedures. However, recent developments are leading to frequent comparisons of pulsed field ablation vs radiofrequency ablation; pulsed field ablation has become an attractive option in some cases over radiofrequency ablation. While radiofrequency ablation […]
From Concept to Market: Understanding FDA Medical Device Product Development

Medical devices are integral to healthcare. Product development must be reliable, safe and effective, but also drive revenue. Therefore, when creating a new device for use in the USA, the FDA-regulated, 5-step medical device product development process must be followed. Knowing how to navigate these steps during the product development project is key […]
The Medical Device Go-To-Market Strategy: The Foundation for Medical Device Success
Bringing a medical device to market is an impressive feat. Strict regulations, fierce competition, and high development costs add complexities to the development journey unseen in other industries. Having a strong development and launch strategy by your side is crucial; a well-defined medical device go-to-market strategy is key to ensure a successful product […]
How to Prevent Component Obsolescence From Becoming Serious Medical Device Obsolescence Risks

The global reliance on electronic components is ever increasing, and consumers today are accustomed to seeing electronic devices become obsolete in less than 2 years. However, for medical devices, the current climate of rapid obsolescence is unacceptable leaving OEMs struggling to manage the resulting medical device obsolescence risks. The unpleasant reality is that when a […]
Effective Tips to Better Manage Medical Device Software Testing

It’s hard to recall a time when software wasn’t standard in electronic medical devices. Creating safe, issue-free software is a critical aspect when developing new devices. As such, it’s no surprise that medical device software testing is vital to a device’s eventual development success and sustainability. Software testing is more involved for medical device software […]
Enhancing Medical Device Cybersecurity Risk Management Through Lifecycle Integration

Medical device development and cybersecurity are crucially linked. Internet of Things (IoT)-enabled devices for diagnostics and patient care are on the rise, as are cyber attacks seeking to leverage their security vulnerabilities. To ensure medical devices don’t create risk for their users, medical device cybersecurity risk management needs to be carefully considered at every stage […]
What Manufacturers Need to Know About Medical Device Software Engineering

Medical device software is a rapidly evolving field. The United States currently leads in this sector, producing 45% of medical device software tools, followed by Germany and South Korea. Regardless of your location, grasping the principles of medical device software engineering is essential for any manufacturing company looking to capitalize on this opportunity. This blog […]
What is an Endoilluminator?
An endoilluminator is the light source that surgeons use to illuminate the inside of a body. Sufficient light is crucial to help surgeons see what they’re doing inside of a body. As such, endoilluminators are a very important piece of equipment. For this reason, medical device companies must treat endoilluminators with the same high regard […]